Digital Therapeutic Exercises Using Augmented Reality Glasses for Frailty Prevention among Older Adults
Article information
Abstract
Objectives
The objective of this study was to investigate the effects of a digital therapeutic exercise platform for pre-frail or frail elderly individuals using augmented reality (AR) technology accessed through glasses. A tablet-based exercise program was utilized for the control group, and a non-inferiority assessment was employed.
Methods
The participants included older adult women aged 65 years and older residing in Incheon, South Korea. A digital therapeutic exercise program involving AR glasses or tablet-based exercise was administered twice a week for 12 weeks, with gradually increasing exercise duration. Statistical analysis was conducted using the t-test and Wilcoxon rank sum test for non-inferiority assessment.
Results
In the primary efficacy assessment, regarding the change in lower limb strength, a non-inferior result was observed for the intervention group (mean change, 5.46) relative to the control group (mean change, 4.83), with a mean difference of 0.63 between groups (95% confidence interval, −2.33 to 3.58). Changes in body composition and physical fitness-related variables differed non-significantly between the groups. However, the intervention group demonstrated a significantly greater increase in cardiorespiratory endurance (p < 0.005) and a significantly larger decrease in the frailty index (p < 0.001).
Conclusions
An ARbased digital therapeutic program significantly and positively contributed to the improvement of cardiovascular endurance and the reduction of indicators of aging among older adults. These findings underscore the value of digital therapeutics in mitigating the effects of aging.
I. Introduction
Individuals aged 65 years and older accounts for 9.3% of the global population [1]. According to the National Statistical Office, South Korea is projected to have the highest proportion of older adults in the world by 2025, at 20.3% [2]. This rapid population aging is particularly notable because with aging, physical and mental capabilities decline substantially, leading to a state of frailty. As frailty progresses, individuals become more susceptible to various illnesses, and overall healthcare costs are adversely impacted [3]. Frailty is specifically characterized by a decline in physiological functions due to aging, lowering the body’s ability to respond to external stressors; this increases the risk of diseases and disabilities [4]. The progression of frailty can also trigger conditions such as diabetes, heart disease, and muscle loss by affecting key systems such as the musculoskeletal, endocrine, respiratory, and cardiovascular systems [5,6]. Preventive strategies for frailty include dietary interventions, lifestyle improvements, and regular exercise. Among these, regular physical activity is a crucial factor for enhancing physical and cognitive functions among older adults [7].
Before the coronavirus disease 2019 (COVID-19) pandemic, most older adults engaged in group-based exercises offered by health and senior support centers [8]. While group exercises have been demonstrated effective in enhancing physical function and alleviating depression, the limitations imposed by factors such as social distancing during large-scale outbreaks of infectious disease (such as COVID-19) can complicate face-to-face group exercises, as can issues related to exercise venue accessibility or adverse weather conditions [9].
Online-based exercise programs for older adults, which are free from time and space constraints, have been introduced as an alternative [10]. Exergames using online platforms, such as CyberCycle, interactive video dance games, and virtual reality (VR) exergames, have been found to improve cognitive functions and help manage depression [11]. However, exergames, including VR programs, can entail side effects such as headaches, dizziness, and fatigue, as well as limited provision of personalized feedback [10]. To address these limitations, remote exercise programs based on video conferencing with real-time feedback can be used. The use of tablet-based video conferencing for exercise programs has been shown to improve skeletal muscle mass, lower limb muscle mass, flexibility, muscle loss, and fall risk factors among older women [12]. However, these exercises involve a fixed field-of-view, which restricts exercise movements and immersion.
Recent advancements in information and communication technology have led to the expansion of augmented reality (AR) technology into the realm of physical exercise. AR offers users intelligent real-world interactions by providing digital information that is otherwise difficult to perceive or utilize in the real world. AR can also be applied in fields such as hospital surgery, medical education programs, and rehabilitation. As such, AR can enhance motivation and encourage continued physical exercise, thus addressing the three key factors in rehabilitation: repetition, rapid feedback, and motivation [13,14].
The field of digital therapeutics (DTx) has recently gained substantial attention. DTx involves evidence-based software-driven behavioral therapy, which aids in preventing and managing medical conditions. Specifically, it leverages digital sensors and wearable devices through the application of VR and AI [15]. DTx facilitates treatment personalization, real-time monitoring of behavioral changes, and prediction of disease exacerbation by stimulating changes in diet, behavior, and physical activity [16]. To date, DTx has demonstrated beneficial impacts regarding the progression of various chronic diseases, including central nervous system disorders such as Parkinson disease as well as diabetes, hypertension, respiratory diseases, musculoskeletal conditions, and lower back pain [17].
The present study involved the development of an AR glasses-based digital therapeutic exercise platform, along with therapeutic exercise content tailored for older adults. We examined the effects of these exercises on physical fitness, body composition, and frailty. Accordingly, we explored the potential of digital therapeutic exercises in preventing frailty.
II. Methods
1. Participants
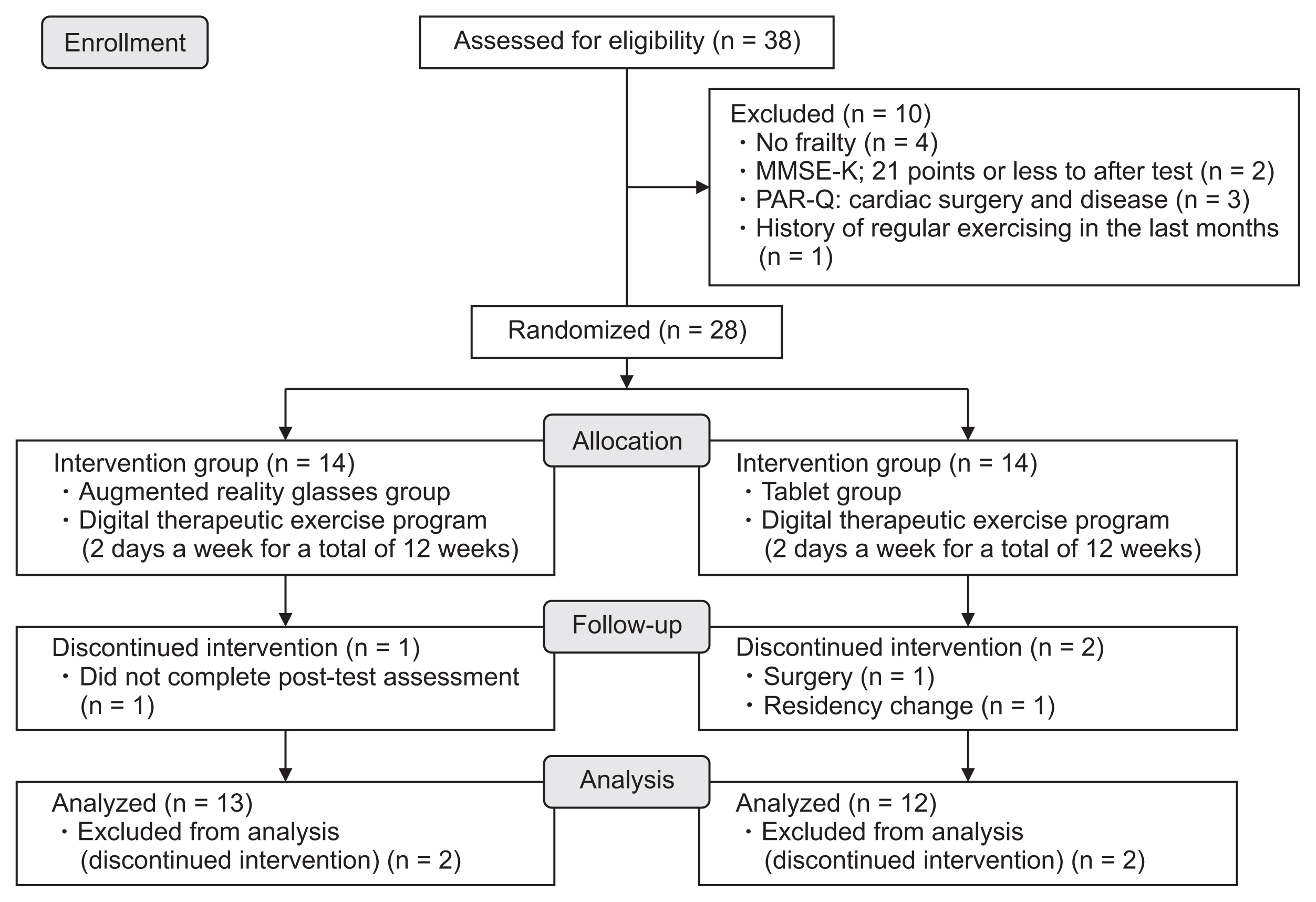
This study was designed as a single-blind, parallel-group, randomized controlled trial. Thirty-one community-dwelling women aged 65 years or older and with pre-frailty or frailty were recruited from Incheon, South Korea. Pre-frailty or frailty was considered to be indicated by a Korean Mini-Mental State Examination score of 21 points or higher. Three women were excluded due to a history of regular exercise for 6 months prior to study initiation (n = 2) and the inability to exercise due to motor dysfunction (n = 1). The remaining 28 participants were assigned to either the exercise intervention group (IG; n = 14) or the control group (CG; n = 14). Three of these 28 participants were excluded from the analysis because they either did not receive the post-test or discontinued the study for medical or personal reasons (Figure 1). The remaining 25 participants completed the study, and their data were included in the analysis. The sample size calculation assumed a mean difference of 0, a standard deviation of the change in measurement of 3.16, and a noninferiority limit of 3. Group allocation was determined by a research coordinator based on simple random sampling, using Microsoft Excel (Microsoft Corp., Redmond, WA, USA). The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Seoul National University Hospital (Approval No. C-2012-139-1183). All participants provided written informed consent prior to enrollment, and they were free to withdraw from the study at any time.
2. Procedure
An in-person assessment of frailty risk factors was conducted 1 week prior to initiation of the intervention. The instructor visited all participants’ homes and provided AR glasses, tablet personal computers (PCs), folding chairs, exercise mats, resistance bands, and smartphone-connected smart bands. The exercises were performed at home using AR glasses for the IG and tablets for the CG participants.
To simplify participation in the exercise program, instructions were provided for operating the AR glasses and tablets. Participants were encouraged to maintain pre-participation physical activity levels throughout the 12-week intervention period. The instructor first recorded a video of the exercise program and later guided participants to watch and follow along with the video. Participants were told to inform the instructor of changes in their rating of perceived exertion (RPE) and real-time heart rate during exercise. A post-test was performed on the day after completion of the 12-week program (Figure 1).
3. Digital Therapeutic Exercise Platform
The digital therapeutic exercise platform comprised two main devices, used for the participants and exercise instructor respectively: AR glasses and an all-in-one PC. The participants utilized AR glasses (Xreal; Matrix Reality Technology Co. Ltd., Beijing, China) connected to a smartphone (LG V50S ThinQ; LG, Seoul, Korea) via a cable, with our WebRTC-based video conferencing system superimposed onto the optical see-through lenses of the device. The AR glasses also provided the IG with video-guided exercise materials. An extra webcam was used to transmit participants’ exercise motions via the conferencing system in real time. The instructor accessed the same video conferencing system via the all-in-one PC, which retrieved, detected, and evaluated the participants’ movements. For deployment, we built a network server for the video conferencing system with an Intel Xeon E-2244G processor 3.8-GHz central processing unit, 16 GB RAM, and an Ubuntu 18.04.5 desktop (64-bit) operating system. The network server met the necessary encryption standards (Hypertext Transfer Protocol Secure, Datagram Transport Layer Security, and Secure Real), in adherence to the Health Insurance Portability and Accountability Act for the security of data transmission of media content and signals. Through the use of Jitsi Meet, this system also ensured the quality of the media content, as well as interoperability among multiple devices (Figure 2).
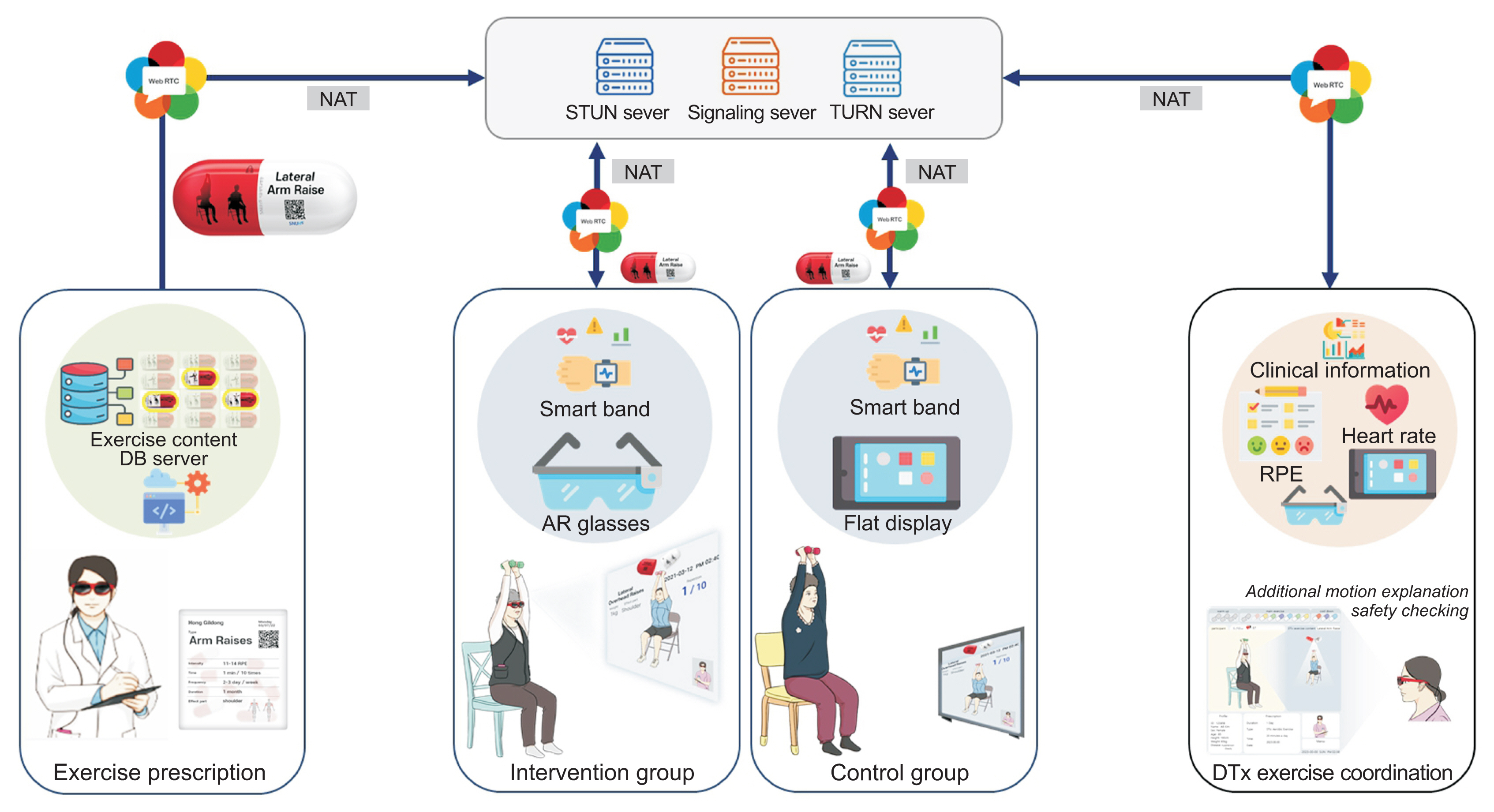
Overview of the digital therapeutic exercise platform using a WebRTC-based video conferencing system superimposed onto the optical see-through lenses of the device. STUN: Session Traversal Utilities for NAT, TURN: Traversal Using Relays around NAT, AR: augmented reality, RPE: rating of perceived exertion, DTx: digital therapeutics, WebRTC: web real-time communication.
4. Digital Therapeutic Exercise Content
During the 12-week exercise session, participants at home turned on the tablet PC and AR glasses, then watched and followed along with exercise videos displayed on these devices. The exercise program was constructed based on the recommendations of the American College of Sports Medicine [18] and on previous studies [17]. Exercises were performed twice per week on non-consecutive days (IG, every Monday and Thursday; CG, every Tuesday and Friday) for 12 weeks. Each session included a warm-up (5 minutes), main exercise (10–30 minutes), and cool-down (5 minutes). Exercise intensity was controlled based on RPE on the Borg scale [19]. The warm-up and cool-down activities involved static and dynamic stretching, as well as comfortable breathing (9 ≤ RPE ≤ 11). The main exercises were resistance exercises, performed using color-coded resistance bands (Thera-Band; Hygenic Corp., Akron, OH, USA) and a chair (11 < RPE ≤ 15). All IG and CG participants performed the exercises during weeks 1–4 without any equipment, for 20 minutes. During weeks 5 to 8, resistance exercises were performed using the red band (1.7 kg) for 30 minutes, and from weeks 9 to 12, a stronger green band (2.1 kg) was used for 40 minutes of resistance exercises. This routine, which targeted the major muscle groups of the arms, shoulders, calves, thighs, hips, and torso, was progressive and increased by 1–2 sets of 10 repetitions per movement. Furthermore, the total exercise time was progressively raised from 20 to 40 minutes over the intervention period. To ensure safety and compliance, exercise training was supervised by professionals with sufficient experience, who served as exercise instructors. All IG and CG participants were provided with the same exercise videos.
5. Outcome Measures
All measurements before and after the intervention were taken by exercise specialists blinded to the group assignment scheme.
1) Body composition
Body composition was evaluated based on the percentage of body fat and skeletal muscle mass, measured using bioelectrical impedance analysis (InBody S610; InBody Co. Ltd., Seoul, South Korea). After height and weight were assessed, four electrodes were attached to both upper and lower extremities with the participant in the supine position. To minimize measurement errors, conditions were systematically assessed prior to measurement. Participants were instructed to adhere to specific guidelines, which included refraining from food and beverage consumption for 4 hours and abstaining from alcohol for 48 hours before measurement. Furthermore, participants were advised to avoid engaging in moderate- to high-intensity physical activity within the prior 12 hours. Additionally, unless prescribed by a physician, the intake of caffeine or diuretics was restricted in preparation for measurement. To ensure accuracy, a visiting technician from the equipment company conducted the examination.
2) Physical function
The Senior Fitness Test (SFT) was used to evaluate physical function [20]. The SFT comprises the chair stand, arm curl, 2-minute step, chair sit-and-reach, back scratch, and 8-foot up-and-go tests. The chair stand test was employed to gauge muscle strength and endurance in the lower body, while the arm curl test was utilized to measure upper body strength. For both assessments, the instructor recorded the number of repetitions completed by the participant within 30 seconds. To assess cardiorespiratory fitness, we employed the 2-minute step test. In this evaluation, the exercise specialist counted the number of complete steps taken by the participant in 2 minutes. A full step was defined as one in which the knee was raised to a point corresponding to the midpoint between the patella and the iliac crest. For lower body flexibility, we utilized the chair sit-and-reach test, while for upper body flexibility, the back-scratch test was used. To evaluate agility and dynamic balance, we employed the 8-foot up-and-go test. In this test, the exercise specialist measured the time it took for the participant to rise to standing from a seated position, walk 8 feet, turn, return to the chair, and resume sitting.
3) Frailty index
The indicators of frailty were sourced from the Cardiovascular Health Study, namely weight loss (at least 4.5 kg over 1 year), poor muscle strength (in the bottom 20% for body mass index [BMI]; having a grip strength below 18 kg for women and below 30 kg for men), emotional fatigue, slow walking speed (in a 4-m walk, if it takes more than 0.8 seconds), and low physical activity (based on the abbreviated International Activity Questionnaire) [5].
6. Statistical Analyses
Demographic analysis was conducted for the IG, while efficacy analysis was performed with the IG as the main analysis group as well as auxiliary to the tablet group. All measured variables were presented as mean ± standard deviation. An independent t-test and the Wilcoxon rank sum test were used to test the homogeneity of the physical characteristics of participants between groups prior to the intervention. A paired t-test and the Wilcoxon rank sum test were used to assess within-group differences between the pre- and post-test measures. The primary efficacy evaluation was conducted under a one-sided test at a significance level of 2.5%, while the secondary efficacy evaluation was performed under a two-tailed test at a significance level of 5%. The significance threshold was p < 0.05 for all tests. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
III. Results
1. Participant Characteristics
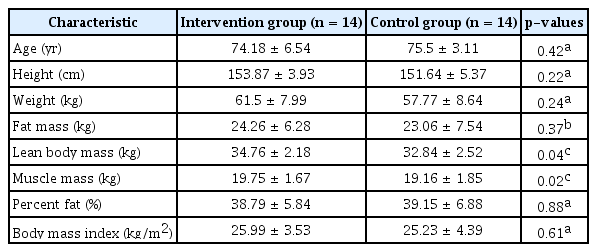
The homogeneity test for physical characteristics and the dependent variables showed that, at baseline, the IG and CG did not differ significantly regarding age, height, weight, fat mass, fat percentage, or BMI. However, differences were observed between lean mass and muscle mass at the 5% significance level. These variables were corrected using a general linear model (Table 1).
2. Primary Outcome
A t-test and Wilcoxon rank sum test were performed 12 weeks after the baseline measurements. These were employed to quantify the change in lower extremity muscle strength for the primary efficacy evaluation, using non-inferiority statistical analysis. The analysis contrasted the IG with the CG approach. The average change in lower extremity muscle strength was 5.46 in the IG and 4.83 in the CG, with an average difference of 0.63 between the groups (95% confidence interval, −2.33 to 3.58). Considering the lower limit of the 95% confidence interval (–2.33), the non-inferiority limit exceeded δ = −3, indicating that the IG was non-inferior to the CG. To correct for the effects of lean and muscle mass, which were significant variables, the estimate of the mean difference between the groups and the 95% confidence interval after correction in the general linear model were 0.23 and (–2.99 to 3.45), respectively, indicating reliability. Because the lower limit of the interval was larger than the noninferiority limit δ = −3, the IG was considered non-inferior to the CG (Table 2).
3. Secondary Outcomes
1) Body composition
Table 3 summarizes the changes in body composition between baseline and the end of the 12-week intervention period. No significant differences were found between groups in body weight (p = 0.66), fat mass (p = 0.60), fat percentage (p = 0.69), muscle mass (p = 0.39), lean body mass (p = 0.44), or BMI (p = 0.42).
2) Physical function
Table 4 displays the changes in physical function between baseline and the end of the 12-week intervention period. No significant differences were observed between the IC and CG in change in the arm curl (p = 0.46), chair sit-and-reach (p = 0.23), back scratch (p = 0.23), or 8-foot up-and-go (p = 0.76) tests. However, the groups differed significantly in the change in the 2-minute step (p < 0.05).
3) Frailty index
Table 5 indicates the changes in the frailty index between baseline and the end of the 12-week intervention period. The two groups differed significantly for this measure (p < 0.01).
IV. Discussion
In the present study, we analyzed the development and application of a digital therapeutic exercise platform and exercise content for older adults in the pre-frail and frail stages. Regarding changes in lower limb strength, the results of the group utilizing AR (the IG) were non-inferior to those of the group administered tablet-based exercise (the CG). Furthermore, the IG exhibited a significantly greater increase in cardiovascular endurance and a significantly larger decrease in indicators of aging.
Lower limb strength among older adults is closely related to physical abilities, which are necessary for daily life activities such as sitting up, walking, and maintaining body balance [21,22]. A decrease in quadriceps femoris muscle strength occurs at a rate of approximately 3% yearly after the age of 50 years, resulting in a decline of 6%–10% over 10 years in middle- aged and older adults [23]. Strength training is crucial for maintaining functional abilities, preventing muscle loss, and avoiding falls [24]. Resistance exercises involving elastic bands have been shown to significantly increase lower limb strength among frail older adults, with a reported increase in lower limb strength after 6 months of resistance exercises for participants with an average age of 82.6 years [25]. Lower limb resistance exercises have been demonstrated to enhance muscle mass and strength among older adults; resistance exercises with elastic bands have shown similar effects to weight training, indicating suitability for this population [26,27]. In our study, resistance exercises with elastic bands were performed twice weekly for 12 weeks. An increase in lower limb strength was observed in both the IG and the CG, with the IG displaying inferior results. Therefore, we propose an AR-based digital therapeutic exercise platform as a new exercise method for counteracting aging.
As aging progresses, the incidence of cardiovascular diseases increases, while diminished cardiovascular endurance is observed due to decreased cardiac output, impairment of skeletal muscle blood flow regulation, and reduced oxygen utilization capacity [28]. This hinders the performance of eating, going out, and other daily life activities for older adults [4]. Previous research has reported significant increases in cardiovascular endurance among older women following 12 weeks of aerobic and resistance exercises performed three times a week, for 60 minutes [4]. In our study, cardiovascular endurance similarly increased in the IG; this was attributed to the progressive increase in exercise intensity every 4 weeks during the 12-week exercise program, as well as the emphasis on breathing during the exercises. Regular exercise intervention is important for mitigating and addressing aging, and it is effective in improving cognitive function and reducing the indicators of aging among older adults [8]. We noted significant improvements in aging indicators, which we attribute to the increase in factors such as physical activity, walking speed, grip strength, and vitality due to the exercise program. However, the significant reduction in the indicators of aging in the IG can be attributed to the unrestricted freedom of movement and wide field-of-view provided by the AR glasses during exercise. Additionally, the large screen and field-of-view helped users immerse themselves in the exercise videos, which may have positively influenced the interactions between the users and the exercise program during the intervention.
We further investigated attendance rates to assess participant compliance. Both groups demonstrated high compliance, with an average attendance rate of over 95% (IG, 95.4%; CG, 95.8%). Therefore, the positive effects observed in this study are likely a result of this high compliance. We believe that the digital therapeutic exercise platform proposed in this study has sufficient value as an exercise program that can be performed regularly, to oppose the effects of aging.
This study had several limitations. First, the sample size was relatively small, and the exclusive focus on women limited generalization of the results. Second, the exercise frequency was low, at twice weekly; the exercise intensity was also relatively low, to ensure participant safety. Considering the high rate of compliance among participants, it may be beneficial to increase the exercise volume in future studies. Finally, a follow-up evaluation is necessary after the 12-week digital therapeutic exercise program to confirm its long-term effects. Future research should include a larger sample size and incorporate quantitative analyses of cost-effectiveness and long-term effectiveness.
Acknowledgments
This work was supported by the Korea Medical Device Development Fund grant funded by the Korean government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety) (Project No. RS-2020-KD000146).
Notes
Conflict of Interest
Hyoun-Joong Kong is an editorial member of Healthcare Informatics Research; however, he did not involve in the peer reviewer selection, evaluation, and decision process of this article. Otherwise, no potential conflict of interest relevant to this article was reported.